Ionic Radius Across Period 3
This increases the overall size of the atom. Na and K are in the same group and K is below Na so K will have higher atomic radius ie.
3 2 Periodic Trends Ib Alchemy
The ionisation energy generally increases across a period.

. Electronegativity in the period table increases as you move from left to right across a period and decreases as you move from top to bottom in a group. For example magnesium atomic weight 243 is placed to the right of sodium atomic weight 230. The gemstones beryl and emerald are both forms of beryllium aluminium silicate Be 3 Al 2 SiO 3 6.
Assume that the current density is uniform throughout the wire. Electrodermal devices that capture the physiological response of skin are crucial for monitoring vital signals but they often require convoluted layered designs with either electronic or ionic. The True Basis of the Periodic Table.
In a period on moving left to right atomic radius decreases. Periodicity of Valence or Oxidation States. In this study COF was evaluated for both B20 ecofuel and diesel using the ASTM D4172 standard for a period of 1 h.
Ionization energy increases as a general trend across the left to right of period 3. Some big hitters - including Mendeleev - were talking seriously about elements lighter than hydrogen and. Periodic Trends and Chemical Reactivity.
As you go down a group the atomic radius increases. In Periods 2 and 3 there are dips at the Group 3A 13 elements boron and aluminium and at the. Arc 30 Buffs and Debuffs.
As you move from left to right across an element period row the ionic radius decreases. However in reality it is not quite so simple. How Many Times Class 3 Notes CBSE Maths.
A long straight wire with 30 A current flowing through it produces magnetic field strength 10 T at its surface. 4What causes the changes in ionic radius from left to right across period 2 of the periodic table. Free NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties solved by expert teachers from latest edition books and as per NCERT CBSE guidelinesClass 11 Chemistry Classification of Elements and Periodicity in Properties NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus.
Electronegativity not only helps us in studying the chemical properties of an atom but also plays a significant role in studying the electron affinity type of bond formed between atoms the magnitude of the bonds. According to Merriam-Webster and the Online Etymology Dictionary the word molecule derives from the Latin moles or small unit of mass. Unlike Radiant on Solar or Voids Invisibility you dont need a certain ability or aspectfragment combo to trigger AmplifyKilling targets with Arc damage while on the new Arc 30 subclasses is enough to become Amplified and this is available across all 3 classes.
DLS is an analytical technique used to measure the particle size distribution of formulations across the oligomer and submicron size ranges of approximately 03 nm to 10 µm. Trends in Physical Properties Atomic Radius Ionic Radius Ionisation Enthalpy Electron Gain Enthalpy Electronegativity Periodic Trends in Chemical Properties. In 1913 chemistry and physics were topsy-turvy.
A vague meaning at first. μ0 4π 10-7 T mA A. Destiny 2 Arc 30 has one major buff for players to tap into.
Even though the size of the atomic nucleus. Sharma in Advances in Eco-Fuels for a Sustainable Environment 2019 1232 Analysis of coefficient of friction. A higher atomic weight than the one on its left.
As you go across a period the atomic radius decreases. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. The chemical symbol for Hydrogen is H.
In February 1798 Vauquelin announced his discovery at the French. As you move from top to bottom down an element group column ionic radius increases. However there are two exceptions to the otherwise smooth increase in the first ionisation energy across periods.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. This is because a new electron shell is added as you move down the periodic table. CH3 CH3 СООН СООН NO2 ŠO3H ŠO3H Identify the reagents represented by the letters in the above reacti.
Since K and Ca are in the same period and K is in 1st group and Ca is in 2nd group so atomic radius of K will be more than Ca ie. Anomalous Properties of Second Period Elements. Molecule 1794 extremely minute particle from French molécule 1678 from New Latin molecula diminutive of Latin moles mass barrier.
So this means that sometimes atoms with. The coefficient of friction COF is one of the key parameters to analyze the tribological characteristics of the tested fuels. The DLS measurements use scattering angles of 90 or 173 degrees using a heliumneon laser as a source of light that is detector position at a back angle 173 degrees and right angle 90 degrees to.
The vogue for the word used until the late 18th century only in Latin. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The French mineralogist Abbé René-Just Haüy thought they might harbour a new element and he asked Nicholas Louis Vauquelin to analyse them and he realised they harboured a new metal and he investigated it.
If the wire has a radius R where within the wire is the field strength equal to 360 of the field strength at the surface of the wire.

Trends In Atomic Radius And Ionic Radius Definition Examples Diagrams

Periodic Trends In Ionic Radii Chemwiki Ionic Radius Ionization Energy Element Chemistry
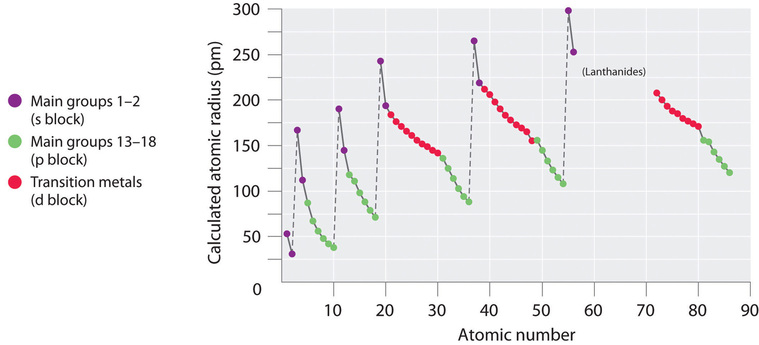
8 2 Atomic And Ionic Radius Chemistry Libretexts

3 2 Trends In Ionic Radii Sl Youtube
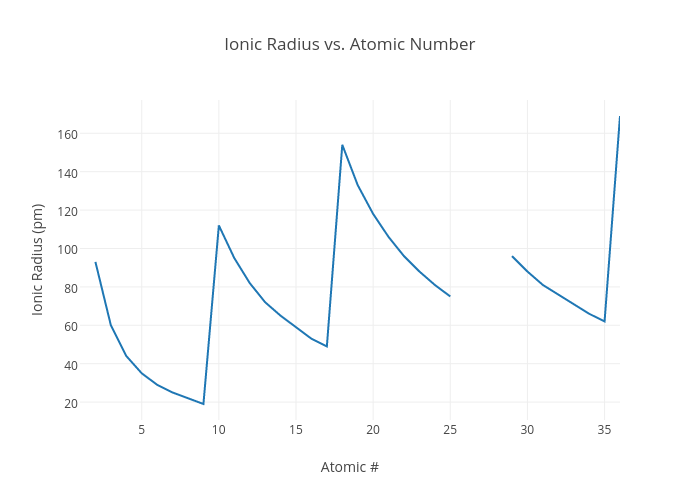
Periodicity Dp Chemistry Ib Recap
0 Response to "Ionic Radius Across Period 3"
Post a Comment